MMI earns FDA clearance for robotic soft tissue dissection tools
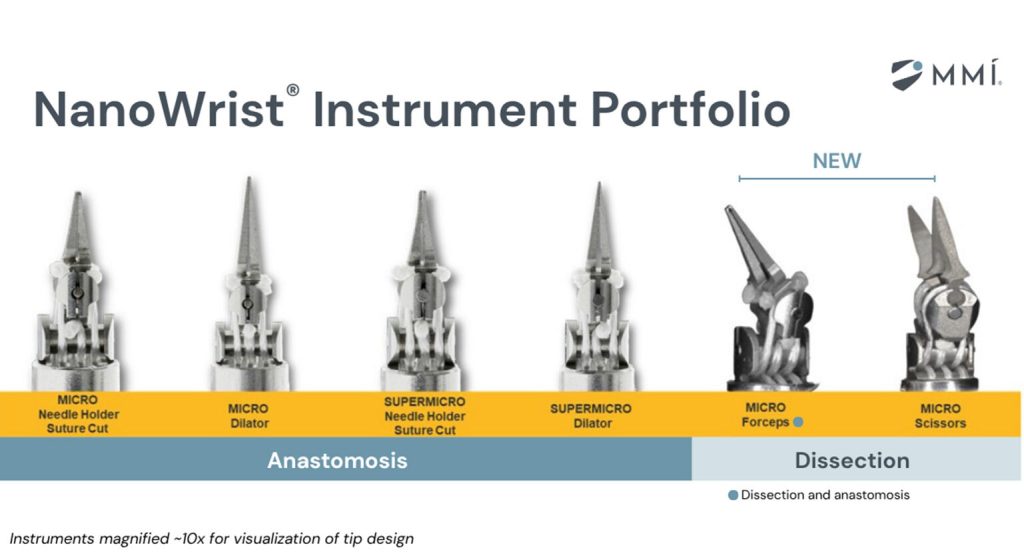
Medical Microinstruments (MMI) has received U.S. Food and Drug Administration 510(k) clearance for its NanoWrist Scissors and Forceps. These instruments are used for soft tissue dissection, allowing MMI’s Symani Surgical System to handle critical steps in complex surgeries.
Surgeons at Tampa General Hospital, which has an established microsurgical robotics program, completed the first U.S. clinical case of the cleared instruments. Surgeons used MMI’s instruments to complete a lymphovenous bypass (LVB) procedure. Symani assisted in the procedure from initial incision to skin closure.
“As we continue to evolve Symani, the first dissection instruments are a critical step in that mission,” said Mark Toland, CEO of MMI. “The addition of these capabilities to our platform is game-changing in supermicrosurgical procedures where extreme precision for the smallest vessels is essential. We will continue to develop technologies that advance the future of microsurgery with Symani and transform microsurgical care and countless lives worldwide.”
MMI’s dissection instruments are some of the smallest fully wristed robotic instruments designed specifically for dissection in open microsurgery. The company designed the NanoWrist Scissors and Forceps to preserve tissue integrity and reduce vessel trauma. This level of precision and control is especially critical in complex procedure, including surgical lymphatic repair, perforator-to-perforator reconstruction, and cancer-related head and neck reconstruction.
New instruments enable Symani to complete specialized surgical techniques
Dr. Nicholas J. Panetta, MD, FACS, chair of the Department of Plastic Surgery at the University of South Florida Morsani College of Medicine and Chief of the Plastic Surgery Institute at TGH, performed the procedure. He specializes in microvascular reconstructive plastic surgery.
For the surgery, Panetta utilized Symani and the NanoWrist Instrument Portfolio to complete a specialized microsurgical technique. This technique redirects tiny lymphatic vessels to nearby veins. This helps relieve swelling by restoring fluid drainage.
He accessed the surgical site and prepared the lymphatic vessels and veins using the NanoWrist Scissors and Forceps. The bypass was then performed with the NanoWrist SuperMicro Needle Holder Suture Cut and SuperMicro Dilator. All instruments in the portfolio offer seven degrees of freedom along with Symani’s motion scaling and tremor reduction features.
“Robotics will define the future of microsurgery, especially in lymphedema work, where sub-millimeter precision is essential. I have been able to push the limits of microsurgery to treat some of the smallest and most delicate vessels, and the impact Symani is having on our patients has been transformative,” shared Dr. Panetta. “With robotic-enabled dissection, I was able to complete a fully robotic LVB, from incision to closure, with unmatched precision, control, and improved ergonomics. These tools are unlocking capabilities that extend beyond the limits of the human hand to support the best possible outcomes for patients.”
MMI makes progress with robotic lymphovenous surgery
Earlier this month, MMI announced that the American Medical Association (AMA) issued a new Current Procedural Terminology (CPT) code for lymphovenous bypass (LVB) surgery. The Centers for Medicare & Medicaid Services (CMS) also finalized and released a payment rate for LVB procedures performed in the outpatient setting to be reflected in the CY 2026 OPPS Final Rule.
Together, these actions create initial reimbursement for a procedure that, until now, required billing under unlisted surgical codes. This makes it easier for U.S. hospitals to submit claims directly related to LVB procedures.
Founded in 2015, MMI said it is developing robotic technology that pushes the limits of soft tissue open surgery. The Pisa, Italy-based company said Symani combines small, wristed micro-instruments with tremor-reducing and motion-scaling technologies.
MMI has made progress in other areas of surgery, including neurosurgery. In October, MMI completed the first cases using its Symani Surgical System in a robotic neurosurgical clinical trial. The Jacobs Institute sponsored the trial. The procedures took place at Buffalo General Medical Center/Gates Vascular Institute, Kaleida Health’s largest facility and hub for heart, vein, and brain care.






Responses